Florida and Texas researchers are developing a new type of battery that is cheaper, safer, and more environmentally friendly than typical Lithium-Ion Batteries. This new sea-water-based battery has a variety of advantages over traditional batteries.
Seawater is so plentiful it covers 71% of the earth’s surface. So unlike lithium, it is easily obtainable. Lithium, on the other hand, has always been the weak link in the battery production chain. Back in 2016, we noted that A Lithium Shortage Could De-Rail The Electric Car Boom.
Since it is plentiful and readily available, a seawater-based battery would cost roughly 1/10th the cost of a lithium battery and, as a bonus, would charge 10x faster than its lithium cousin.
But cost, charging time, and availability are not the only problems facing Lithium batteries.
Lithium Battery Problems
When we think of problems with Lithium batteries, the first thing that jumps to mind is often the flammability issue. Recently, a video made a Social Media sensation as a gator ate a drone. But the worst part wasn’t the loss of the drone but the fact that it contained a Lithium battery that burst into flames as the gator munched on it. The following video shows the smoke emanating from the gator.
At one point, battery flammability was such a serious issue that the FAA banned certain brands of laptops from planes because they were especially susceptible to battery fire issues. Plus, if the prospect of flames on a plane isn’t enough… as it burns, a lithium battery gives off toxic fumes, which is certainly not something you want to happen in a closed environment.
In addition to the fire issues, mining Lithium itself is an environmental disaster. Lithium extraction requires approximately 500,000 gallons per metric ton of lithium produced. First, they drill a hole into the salt flats; then, they pump in water—eventually, a salty, mineral-rich brine bubbles to the surface. The water in the brine is then allowed to evaporate, leaving behind a mixture of manganese, potassium, borax, and lithium salts. At that point, it is filtered and placed into another evaporation pool. At which point, it takes another 12 to 18 months before the lithium carbonate can be extracted.
Recycling
To reduce the limited recycling of lithium, in early 2021, Canadian firm Li-Cycle announced the construction of a US $175 million Lithium recycling plant in Rochester, N.Y., on the old Kodak campus. But recycling has its risks, with several fires occurring at other recycling facilities. Lithium is typically stored in protective oils that keep it from bursting into flames. So if the oil is removed during the recycling process, it can become dangerous.
If unwanted items like laptops end up in landfills, hazardous chemicals from the electrodes and toxic ionic fluids from the electrolyte can leak into the environment or cause landfill fires.
Saltwater Battery
For ages, science has known that saltwater conducts electricity. So researchers have sought to be able to use it as the battery electrolyte, but there were problems, including the growth of dendrites on the electrodes. The seawater battery has overcome many of these limitations and could provide a better electrolyte because of the seawater’s additional minerals (besides just salt).
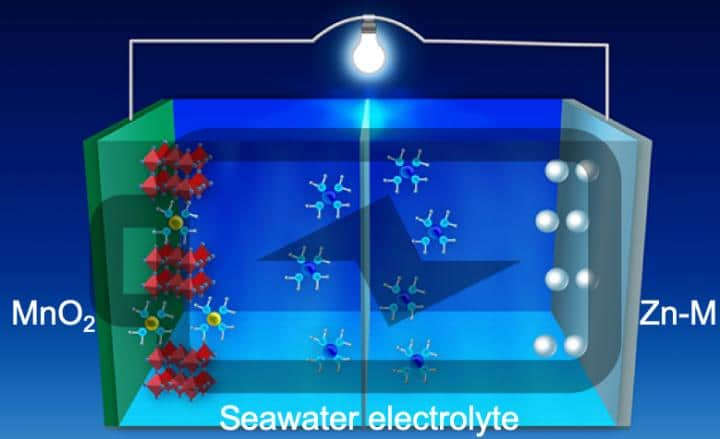
Seawater Battery:
Researchers from the University of Central Florida (UCF) NanoScience Technology Center, in conjunction with researchers from the electrical and computer engineering center at the University of Houston, have developed a “wet cell” battery that uses filtered seawater as its electrolyte rather than the toxic chemicals in lithium batteries. Even the old-style lead-acid car batteries were more toxic than seawater.
The researchers claim that the new seawater based battery is “a low-cost, high energy density, stable battery” that should result in a reliable, rechargeable battery.
Development Issues:
One of the problems with water-based zinc batteries is that the zinc-based electrodes would grow dendrites on the internal zinc anode. This dendrite growth rapidly reduced their lifespan and degraded their performance, making them unsuitable for practical applications. To address this problem, the researchers in Texas developed a unique optical visualization technique that allowed them to see the reaction dynamics on the anode in real time. By observing the dendrite growth, they were able to develop a new 3D zinc-manganese nano-alloy anode.
This new type of anode has overcome the previous limitations, resulting in a stable, high-performance, dendrite-free aqueous battery using seawater as the electrolyte.
The UCF researcher, Feng, says they used seawater as the battery electrolyte or chemical medium that allows the electrical charge to flow between anode and cathode because of its abundance and potential use in deep-sea energy storage applications. The research was funded primarily by the National Science Foundation. Other researchers from Pacific Northwest National Laboratory, Oregon State University, and Argonne National Laboratory also assisted.
IBM’s Seawater Based Battery
On a similar note, IBM claims to have developed their own Seawater battery. Looking at the limited IBM information, they aren’t using seawater as the electrolyte. Instead, they are extracting minerals from seawater for use in their battery.
You might also like:
Lithium Articles:
- Move Over Oil – Lithium Is The Future
- Could A Lithium Shortage De-Rail The Electric Car Boom?
- A New Lithium War Is About To Begin
- Move Aside Lithium – Vanadium Is The New Super-Metal
Battery Articles:
